Aktuelles
WPT in der Schweißen und Schneiden!
Ausgabe zum Schwerpunktthema Werkstoffprüfung

Nachhaltige Wasserstoffforschung mit der Universität von Alberta in Kanada
Der Lehrstuhl für Werkstoffprüftechnik begrüßt Assistenz-Professor Mostafa Yakout vom Fachbereich Maschinenbau der University of Alberta, um über…

Tag der offenen Tür 2023 an der TU Dortmund
Laborführungen mit Live-Experimenten

Vierte Vollversammlung der DFG-Forschungsgruppe 5250 zu permanenten und bioresorbierbaren Implantaten
Patient*innenspezifische, additiv-gefertigte Implantate mit maßgeschneiderter Funktionalität für die Mund-, Kiefer- und Gesichtsheilkunde.

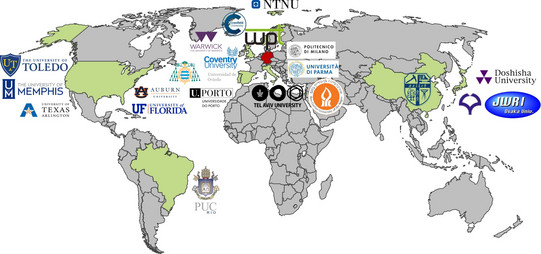
Internationale Kooperationen
Das WPT stellt sich vor
Bitte bestätigen Sie die Aktivierung dieses Videos.
Nach der Aktivierung werden Cookies gesetzt und Daten an YouTube (Google) übermittelt.
Zur Datenschutzerklärung von Google